In Science the Word Light Refers to What
What Is Visible Light?
Visible light is a form of electromagnetic (EM) radiation, as are radio waves, infrared radiation, ultraviolet radiation, X-rays and microwaves. Generally, visible light is defined as the wavelengths that are visible to most human eyes.
EM radiation is transmitted in waves or particles at different wavelengths and frequencies. This broad range of wavelengths is known as the electromagnetic spectrum. That spectrum is typically divided into seven regions in order of decreasing wavelength and increasing energy and frequency. The common designations are radio waves, microwaves, infrared (IR), visible light, ultraviolet (UV), X-rays and gamma-rays.
Visible light falls in the range of the EM spectrum between infrared (IR) and ultraviolet (UV). It has frequencies of about 4 × 1014 to 8 × 1014 cycles per second, or hertz (Hz) and wavelengths of about 740 nanometers (nm) or 2.9 × 10−5 inches, to 380 nm (1.5 × 10−5 inches).
Color
Perhaps the most important characteristic of visible light is color. Color is both an inherent property of light and an artifact of the human eye. Objects don't "have" color, according to Glenn Elert, author of the website The Physics Hypertextbook. Rather, they give off light that "appears" to be a color. In other words, Elert writes, color exists only in the mind of the beholder.
Our eyes contain specialized cells, called cones, that act as receivers tuned to the wavelengths of this narrow band of the EM spectrum, according to NASA's Mission Science website. Light at the lower end of the visible spectrum, having a longer wavelength, about 740 nm, is seen as red; light in the middle of the spectrum is seen as green; and light at the upper end of the spectrum, with a wavelength of about 380 nm, is seen as violet. All other colors that we perceive are mixtures of these colors.
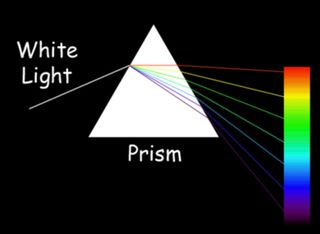
For instance, yellow contains both red and green; cyan is a mixture of green and blue, and magenta is blend of red and blue. White light contains all colors in combination. Black is a total absence of light. The first person to realize that white light was made up of the colors of the rainbow was Isaac Newton, who in 1666 passed sunlight through a narrow slit and then a prism to project the colored spectrum onto a wall, according to Michael Fowler, a physics professor at the University of Virginia.
Color and temperature
As objects grow hotter, they radiate energy dominated by shorter wavelengths, which we perceive as changing colors, according to NASA. For example, the flame of a blowtorch changes from reddish to blue as it is adjusted to burn hotter. This process of turning heat energy into light energy is called incandescence, according to the Institute for Dynamic Educational Advancement's website, WebExhibits.org.
Incandescent light is produced when hot matter releases a portion of its thermal vibration energy as photons. At about 800 degrees Celsius (1,472 degrees Fahrenheit), the energy radiated by an object reaches the infrared. As the temperature increases, the energy moves into the visible spectrum and the object appears to have a reddish glow. As the object gets hotter, the color changes to "white hot" and eventually to blue.
Visible light astronomy
The color of hot objects, such as stars, can be used to estimate their temperatures, according to IDEA. For example, the sun's surface temperature is about 5,800 Kelvin (9,980 F or 5,527 C). The light emitted has a peak wavelength of about 550 nm, which we perceive as visible white light (or slightly yellowish).
According to NASA, if the sun's surface temperature were cooler, about 3,000 C, it would look reddish, like the star Betelgeuse. If it were hotter, about 12,000 C, it would look blue, like the star Rigel.
Astronomers can also determine what objects are made of because each element absorbs light at specific wavelengths, called an absorption spectrum. By knowing the absorption spectra of elements, astronomers can use spectroscopes to determine the chemical composition of stars, dust clouds and other distant objects.
Additional resources
- NASA Mission Science: Visible Light
- The Physics Hypertextbook: Color
- WebExhibits.org: Causes of Color

Jim Lucas is a contributing writer for Live Science. He covers physics, astronomy and engineering. Jim graduated from Missouri State University, where he earned a bachelor of science degree in physics with minors in astronomy and technical writing. After graduation he worked at Los Alamos National Laboratory as a network systems administrator, a technical writer-editor and a nuclear security specialist. In addition to writing, he edits scientific journal articles in a variety of topical areas.
In Science the Word Light Refers to What
Source: https://www.livescience.com/50678-visible-light.html